Electron Machine Corporation engaged F2 Labs for the technical assessment of the MPR E-Scan using the European standard, EN 50581:2012 Technical documentation for the assessment of electrical and electronic products with respect to the restriction of hazardous substances, to determine compliance with the RoHS Directive 2011/65/EU, and has determined Electron Machine Corporation can claim compliance to the RoHS Directive 2011/65/EU for the subject equipment based upon the supplied documentation.
RoHS stands for Restriction of Hazardous Substances. RoHS, also known as Directive 2002/95/EC, originated in the European Union and restricts the use of specific hazardous materials found in electrical and electronic products.
This certification applies to each portion of the MPR E-Scan (IS), which includes the Console, Cable, Barrier Box, and Sensing Head; along with all components that each of these major subassemblies of the MPR E-Scan contain.
For more information, please contact Electron Machine at 352-669-3101 or by visiting this link.
This blog focuses on industrial, inline process refractometers and their use in industrial applications. Refractometry is used to measure the refractive index of a substance in order to determine its composition or purity. Posts include information on theory, construction, installation, new products and new markets.
Electron Machine Corporation | Umatilla, FL | PHONE: 352-669-3101 | ElectronMachine.com
Paper Manufacturing: Kraft (Sulfate) Pulping
Abstracted from Washington State Air Toxic Sources
and Emission Estimation Methods
and Emission Estimation Methods
Pulping is the term used for the process which separates wood fibers. Chemical pulping, dissolving the lignin in the wood to create a pulp, is the most commonly used pulping process. Chemical pulping creates higher sheet strength than mechanical pulping; however, yields 40 to 50 percent pulp, where mechanical pulping yields 95 percent pulp.
The two main types of chemical pulping are the more common sulfate pulping (most commonly known as Kraft pulping) and sulfite pulping. Kraft pulping accommodates a variety of tree species, recovers and reuses all pulping chemicals, and creates a paper with a higher sheet strength. Sulfite pulp, however, is easier to bleach, yields more bleached pulp, and is easier to refine for papermaking. The major difference between the two types of chemical pulping is the types of chemicals used to dissolve the lignin.
The Kraft process was developed in Germany in 1879 and was first applied to a Swedish mill in 1885. The resulting paper was much stronger than any paper previously made, and therefore the process was named “Kraft”, (German and Swedish for “strength”). Kraft pulping creates dark brown paper which is used for boxes, paper bags, and wrapping paper. Kraft pulp can also be used for writing paper and paperboard when bleached, and for diapers when fluffed.
The three main steps involved in Kraft pulping are:
- Digestion: wood chips are cooked
- Washing: black liquor is separated from the pulp
- Chemical recovery: chemicals are recovered from the black liquor for reuse
- Digestion
Relief gases are vented continuously from the digester, which helps remove air and other non- condensable gases and reduce the pressure at blow, when the pulp is discharged to the blow tank. After the cooking process, the pulp and black liquor (the chemical mix left after the cooking process) are discharged to a blow tank.
By-products can be recovered from the digestion process. For example, turpentine distills with water out of the blow tank and the evaporators and is separated to be used. The resin acids and fatty acids dissolved from the wood form sodium soaps which are skimmed off the black liquor from storage tanks, evaporators, and black liquor oxidation tanks, and then acidified with sulfuric acid to form tall oil.
Before the washing process, the pulp is usually sent to deknotters, screens used to remove knots (large pieces of fiber not completely broken down in the digester).
- Brownstock Washing
All the washer types use water (fresh or recycled) and are usually placed in series to achieve higher removal efficiency.
The rinsed pulp is screened for oversize particles and then excess water is removed. This is done in a gravity thickener (more commonly known as a decker).
- Chemical Recovery
The first step in recovering the chemicals from the black liquor is evaporation. This removes excess water from the black liquor and maximizes the fuel value for the recovery furnace.
There are two types of evaporators generally used in the chemical recovery process: direct (DCE) and indirect (NDCE) contact evaporators. Some types of DCE include the multiple-effect evaporator (most common), flash evaporation and thermocompressor evaporation. DCE use heat from direct contact with the recovery furnace flue gases, while NDCE uses indirect contact.
Black liquor oxidation is needed after DCE, but not after NDCE. After DCE, the black liquor is normally oxidized with air to control the sulfide level and prevent the release of odorous compounds. This is done by countercurrently passing the black liquor through an air stream using a porous diffuser, sieve tray tower, packed tower or agitated air sparge. The oxidation reaction converts sodium sulfide to sodium thiosulfate.
After NDCE or black liquor oxidation, the black liquor is then forced through spray nozzles into the recovery furnace, where it is burned providing heat to generate steam. This also conserves the inorganic chemicals, which create a molten smelt on the floor of the furnace.
The molten smelt, composed of sodium sulfide and sodium carbonate, is drained from the recovery furnace hearth through smelt spouts. In a smelt dissolving tank, the smelt is quenched with water, producing green liquor.
Sodium carbonate from the smelt is then converted to sodium hydroxide in the causticizer by adding calcium hydroxide. The calcium carbonate resulting from the reaction precipitates from the solution and is collected and sent to the lime kiln where it is converted to lime (calcium oxide). The calcium oxide is then slaked to produce calcium hydroxide for reuse in the causticizer.
Hybrid-Digital Process Refractometer
Process refractometers are used for monitoring, controlling, and recording the concentration of dissolved solids in a process media. They accurately measure refractive index and temperature of the process media and provide a visual display in units specific to that process (i.e. Brix, Percent Solids, Dissolved Solids, SGU, R.I.).
The MPR E-Scan is a hybrid-digital critical angle refractometer. It is used to measure the refractive index of process fluids and may be used as an error indicator or an integral part of a complete process control system.
The MPR E-Scan is calibrated and temperature compensated to your process specifications. It is ready for installation and immediate use when received. Calibration procedures are available to change system parameters and allow the refractometer to measure different process fluids.
How It Works: Hybrid-Digital Measurement
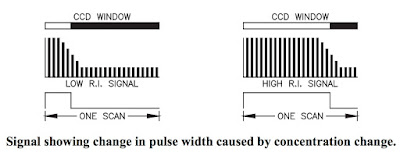
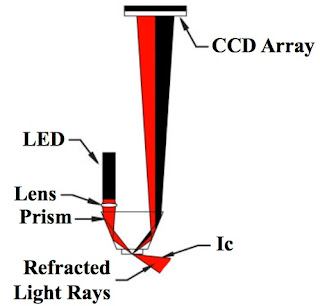
 Energy radiated from the LED passes through the prism surface to be reflected off a mirror to the prism-to-process interface. The light reaching this interface intersects the same interface over a series of angles specifically chosen to include the critical angle (Ic) for the process being measured. Light intersect- ing the interface at an angle greater than critical angle is refracted into the solution. Light intersecting the interface at less than critical angle is reflected up out of the prism up to the digital CCD linear array to be scanned.
Energy radiated from the LED passes through the prism surface to be reflected off a mirror to the prism-to-process interface. The light reaching this interface intersects the same interface over a series of angles specifically chosen to include the critical angle (Ic) for the process being measured. Light intersect- ing the interface at an angle greater than critical angle is refracted into the solution. Light intersecting the interface at less than critical angle is reflected up out of the prism up to the digital CCD linear array to be scanned.The resolution of each sensing head is maximized by selecting the angle of the prism for the measurement and temperature range of the process.
 |
| MPR E-Scan |
Isolation Valve Adapter and Safeguard Tool Demonstration
Plant personnel safety is extremely important to Electron Machine. Our Isolation Valve Adapter has a proven track record for safety and reliability for safe removal of our process refactometer sensing heads from a pressurized pipeline. Continuing toward our goal for absolute safety, EMC designed and developed the EMC Safeguard Tool, a device designed to further increase safety should abnormal situations arise when removing a sensing head from the process pipe line. Check out the demonstration below for a full understanding.
For more information, visit http://www.electronmachine.com or call 352-669-3101.
For more information, visit http://www.electronmachine.com or call 352-669-3101.
HART Communication Protocol
 |
| (Image courtesy of Lessons in Industrial Instrumentation and Tony R. Kuphaldt and shared under Creative Commons 4.0 International Public License). |
HART instruments have the capacity to perform in two main modes of operation: point to point, also known as analog/digital mode, and multi-drop mode. The point to point mode joins digital signals with the aforementioned 4-20 mA current loop in order to serve as signal protocols between the controller and a specific measuring instrument. The polling address of the instrument in question is designated with the number ì0î. A signal specified by the user is designated as the 4-20 mA signal, and then other signals are overlaid on the 4-20 mA signal. A common example is an indication of pressure being sent as a 4-20 mA signal to represent a range of pressures; temperature, another common process control variable, can also be sent digitally using the same wires. In point to point, HART’s digital instrumentation functions as a sort of digital current loop interface, allowing for use over moderate distances.
HART in multi-drop mode differs from point to point. In multi-drop mode, the analog loop current is given a fixed designation of 4 mA and multiple instruments can participate in a single signal loop. Each one of the instruments participating in the signal loop need to have their own unique address.
Since the HART protocol is a standardized process control industry technology, each specific manufacturer using HART is assigned a unique identification number. This allows for devices participating in the HART protocol to be easily identified upon first interaction with the protocol. Thanks to the open protocol nature, HART has experienced successive revisions in order to enhance the performance and capabilities of the system relating to process control. The standardization of “smart” implementation, along with the ability to function with the legacy 4-20 mA technology and consistent development, has made HART a useful and popular component of the process measurement and control industry framework.
Overview of Chemical Recovery Processes in Pulp & Paper Mills
 |
| Figure 1 |
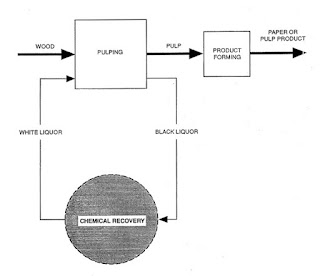
The production of kraft and soda paper products from wood can be divided into three process areas:
- Pulping of wood chips
- Chemical recovery
- Product forming (includes bleaching)
 |
| Figure 2 |
The purpose of the chemical recovery cycle is to recover cooking liquor chemicals from spent
cooking liquor. The process involves concentrating black liquor, combusting organic compounds, reducing inorganic compounds, and reconstituting cooking liquor.
Cooking liquor, which is referred to as "white liquor, is an aqueous solution of sodium hydroxide (Na01) and sodium sulfide (Na2S) that is used in the pulping area of the mill. In the pulping process, white liquor is introduced with wood chips into digesters, where the wood chips are "cooked" under pressure. The contents of the digester are then discharged to a blow tank, where the softened chips are disintegrated into fibers or "pulp. The pulp and spent cooking liquor are subsequently separated in a series of brown stock washers: Spent cooking liquor, referred to as "weak black liquor, from the brown stock washers is routed to the chemical recovery area. Weak black liquor is a dilute solution (approximately 12 to 15 percent solids) of wood lignins, organic materials, oxidized inorganic compounds (sodium sulfate (Na2SO4), sodium carbonate (Na2003)), and white liquor (Na2S and Na0H).
In the chemical recovery cycle, weak black liquor is first directed through a series of multiple-effect evaporators (MEE's) to increase the solids content to about 50 percent. The "strong. (or "heavy") black liquor from the MEE's is then either oxidized in the BLO system if it is further concentrated in a DCE or routed directly to a concentrator (NDCE). Oxidation of the black liquor prior to evaporation in a DCE reduces emissions of TRS compounds, which are stripped from the black liquor in the DCE when it contacts hot flue gases from the recovery furnace. The solids content of the black liquor following the final evaporator/concentrator typically averages 65 to 68 percent.
Concentrated black liquor is sprayed into the recovery furnace, where organic compounds are combusted, and the Na2SO4 is reduced to Na2S. The black liquor burned in the recovery furnace has a high energy content (13,500 to 15,400 kilojoules per kilogram (kJ/kg) of dry solids (5,800 to 6,600 British thermal units per pound {Btu/lb} of dry solids)), which is recovered as steam for process requirements, such as cooking wood chips, heating and evaporating black liquor, preheating combustion air, and drying the pulp or paper products. Particulate matter (PM) (primarily Na2SO4) exiting the furnace with the hot flue gases is collected in an electrostatic precipitator (ESP) and added to the black liquor to be fired in the recovery furnace. Additional makeup Na2SO4, or "saltcake," may also be added to the black liquor prior to firing.
Molten inorganic salts, referred to as "smelt," collect in a char bed at the bottom of the furnace. Smelt is drawn off and dissolved in weak wash water in the SDT to form a solution of carbonate salts called "green liquor," which is primarily Na2S and Na2CO3. Green liquor also contains insoluble unburned carbon and inorganic Impurities, called dregs, which are removed in a series of clarification tanks.
Decanted green liquor is transferred to the causticizing area, where the Na2CO3 is converted to NaOH by the addition of lime (calcium oxide [Ca0]). The green liquor is first transferred to a slaker tank, where Ca0 from the lime kiln reacts with water to form calcium hydroxide (Ca(OH)2). From the slake, liquor flows through a series of agitated tanks, referred to as causticizers, that allow the causticizing reaction to go to completion (i.e., Ca(OH)2 reacts with Na2CO3 to form NaOH and CaCO3).
The causticizing product is then routed to the white liquor clarifier, which removes CaCO3 precipitate, referred to as "lime mud." The lime mud, along with dregs from the green liquor clarifier, is washed in the mud washer to remove the last traces of sodium. The mud from the mud washer is then dried and calcined in a lime kiln to produce "reburned" lime, which is reintroduced to the slaker. The mud washer filtrate, known as weak wash, is used in the SDT to dissolve recovery furnace smelt. The white liquor (NaOH and Na2S) from the clarifier is recycled to the digesters in the pulping area of the mill.
At about 7 percent of kraft mills, neutral sulfite semi-chemical (NSSC) pulping is also practiced. The NSSC process involves pulping wood chips in a solution of sodium sulfite and sodium bicarbonate, followed by mechanical de-fibrating. The NSSC and kraft processes often overlap in the chemical recovery loop, when the spent NSSC liquor, referred to as "pink liquor," is mixed with kraft black liquor and burned in the recovery furnace. In such cases, the NSSC chemicals replace most or all of the makeup chemicals. For Federal regulatory purposes, if the weight percentage of pink liquor solids exceeds 7 percent of the total mixture of solids fired and the sulfidity of the resultant green liquor exceeds 28 percent, the recovery furnace is classified as a "cross-recovery furnace.'" Because the pink liquor adds additional sulfur to the black liquor, TRS emissions from cross recovery furnaces tend to be higher than from straight kraft black liquor recovery furnaces.
Industrial Inline Refractometers for Sucrose, Fructose and Dextrose
The Electron Machine MPR E-Scan is perfectly suited for sugar applications. The refractometer directly measures dissolved solids, which can be easily converted to Brix.
In sugar refineries, the MPR E-Scan can be used to monitor and control Brix measurement from the beginning of the evaporation stages up to the seed point of crystallization.
The suggested adapter for most installations is either a 316S/S in-line type adapter or a 316S/S vacuum pan adapter. If coating may be an issue, a steam or hot water wash nozzle can be provided.
Sanitary-type adapters designed and manufactured to appropriate 3-A Sanitary Standards are also available if needed.
In sugar refineries, the MPR E-Scan can be used to monitor and control Brix measurement from the beginning of the evaporation stages up to the seed point of crystallization.
The suggested adapter for most installations is either a 316S/S in-line type adapter or a 316S/S vacuum pan adapter. If coating may be an issue, a steam or hot water wash nozzle can be provided.
Sanitary-type adapters designed and manufactured to appropriate 3-A Sanitary Standards are also available if needed.
Subscribe to:
Posts (Atom)